Tarak Dhurjati
Exosomes—microscopic vesicles released naturally by almost every cell in the human body—are reshaping the future of medicine. Measuring just 30–150 nanometers, these nano-sized biological couriers transport proteins, lipids, and RNA between cells, enabling communication, repair, and immune modulation. Their natural stability and compatibility with human tissues make them ideal candidates for delivering therapies directly to diseased organs with far greater precision than traditional drugs. Unlike synthetic nanoparticles, exosomes are not foreign to the body. They move seamlessly through tissues, protect their cargo from degradation, and can even cross highly restrictive barriers such as the blood–brain barrier. This unique set of advantages has fueled a wave of scientific innovation aimed at transforming exosomes into programmable delivery vehicles for modern therapeutics.
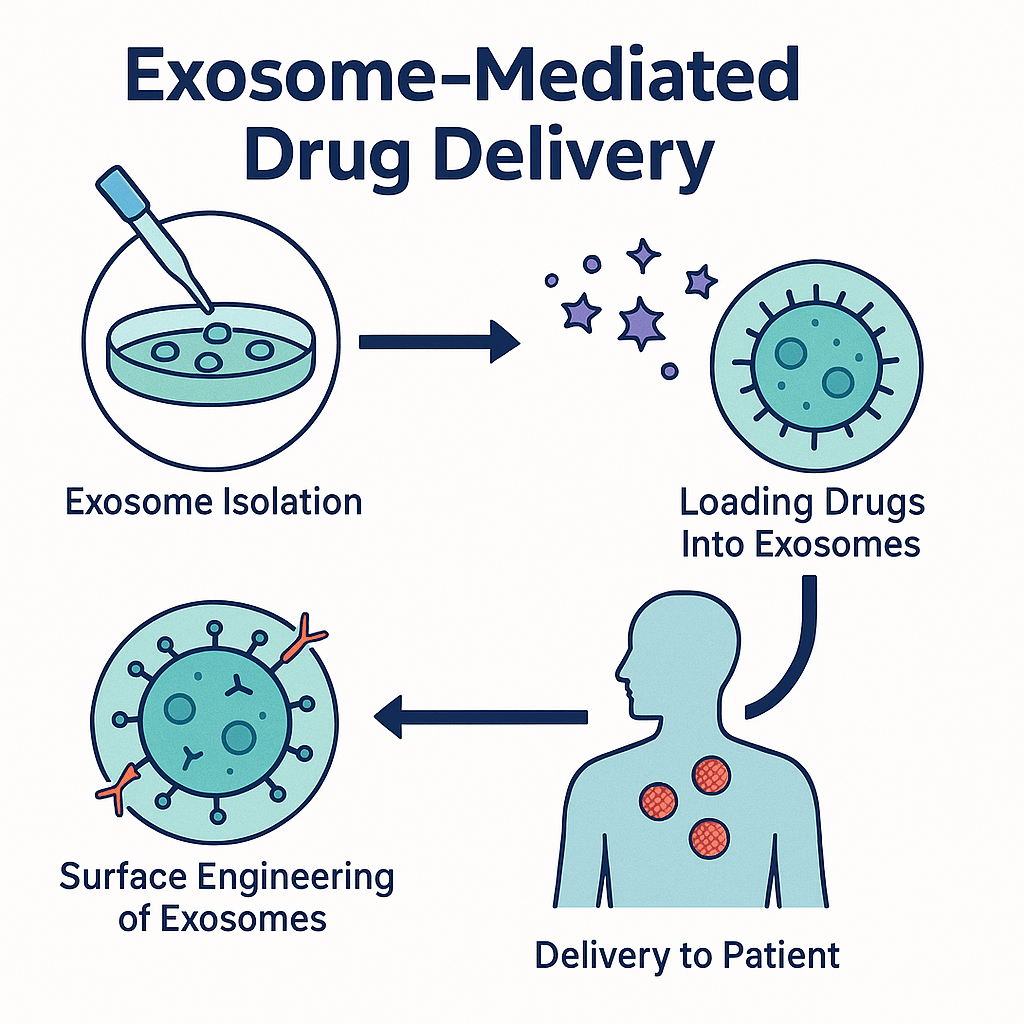
In oncology, exosomes are being engineered to carry chemotherapy agents straight into tumors, reducing systemic toxicity and sparing healthy tissues. Neurology is another field seeing rapid progress: exosomes loaded with RNA or small molecules can travel into the brain to target Alzheimer’s, Parkinson’s disease, and stroke-damaged tissue—an area where most conventional drug systems fail. Exosomes derived from stem cells are also being explored for regenerative medicine, where they promote tissue repair, reduce inflammation, and accelerate healing without the risks associated with transplanting whole living cells. This has opened new possibilities for cartilage restoration in osteoarthritis, regeneration of heart muscle after myocardial infarction, and rapid wound repair in surgical and skin-injury settings.
Engineered Exosomes: The Future of Precision Drug Delivery
As the field advances, scientists have moved from simply harvesting natural exosomes to designing engineered exosomes—custom-built biological nanoparticles with enhanced therapeutic power. Engineering occurs in two main dimensions: cargo engineering and surface engineering. Cargo engineering enables drugs, siRNA, mRNA, CRISPR components, or therapeutic proteins to be packed inside exosomes using technologies such as electroporation, sonication, extrusion, or pre-programming donor cells so they naturally load therapeutic molecules during exosome formation.
Surface engineering adds a second level of precision. By attaching targeting ligands such as antibodies, peptides, aptamers, or receptor-binding proteins onto the exosome membrane, scientists can direct exosomes to highly specific tissues—tumor microenvironments, inflamed joints, heart muscle, or neural tissue. The result is a delivery platform capable of navigating complex biological terrain and releasing therapies exactly where they are needed, minimizing off-target toxicity and maximizing therapeutic efficiency.
This advancement represents more than a new drug-delivery tool; it is the emergence of programmable biological nanocarriers, bridging the gap between synthetic nanotechnology and living cellular therapy.
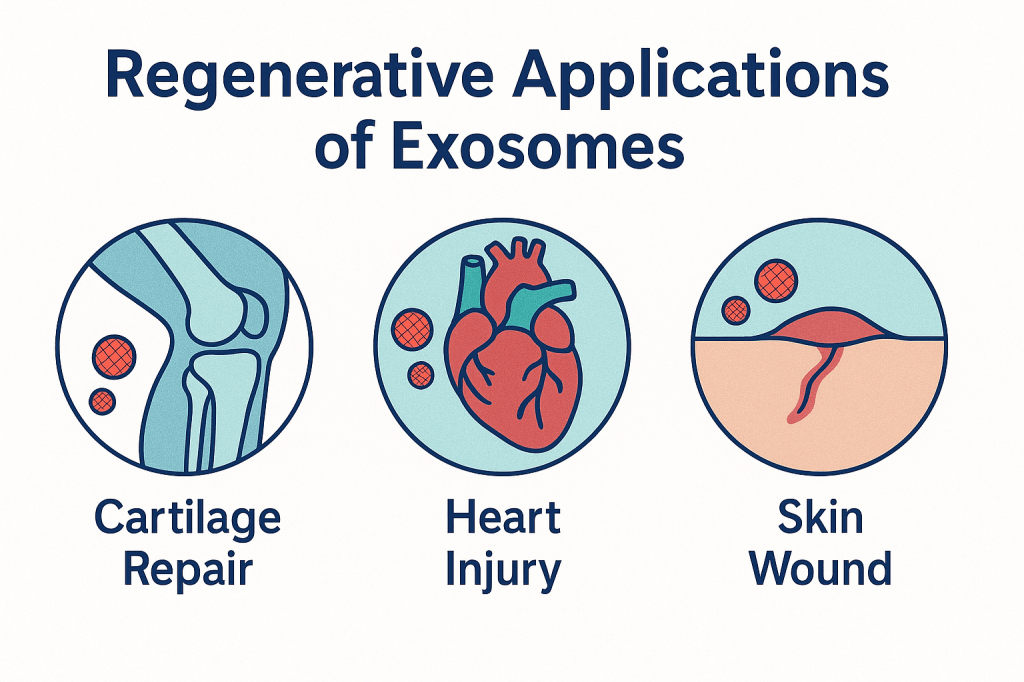
Regenerative Medicine: Healing From Within
Stem-cell-derived exosomes have become a cornerstone of next-generation regenerative medicine. Instead of transplanting entire stem cells—a process that carries risks of immune rejection or unwanted cell differentiation—researchers can isolate the exosomes those cells produce and use them as safe, potent biological repair kits.
In cartilage repair, exosomes stimulate chondrocytes and reduce inflammation, offering promise for osteoarthritis patients seeking alternatives to surgery. In cardiology, exosomes released from mesenchymal stem cells promote angiogenesis, protect surviving heart cells, and improve functional recovery after heart attacks. Dermatology and wound care are also major beneficiaries, with exosomes accelerating tissue regeneration in burns, diabetic ulcers, and surgical wounds. Their ability to coordinate healing makes them a powerful, cell-free regenerative modality.
Exosomes in Therapeutics Across Medicine
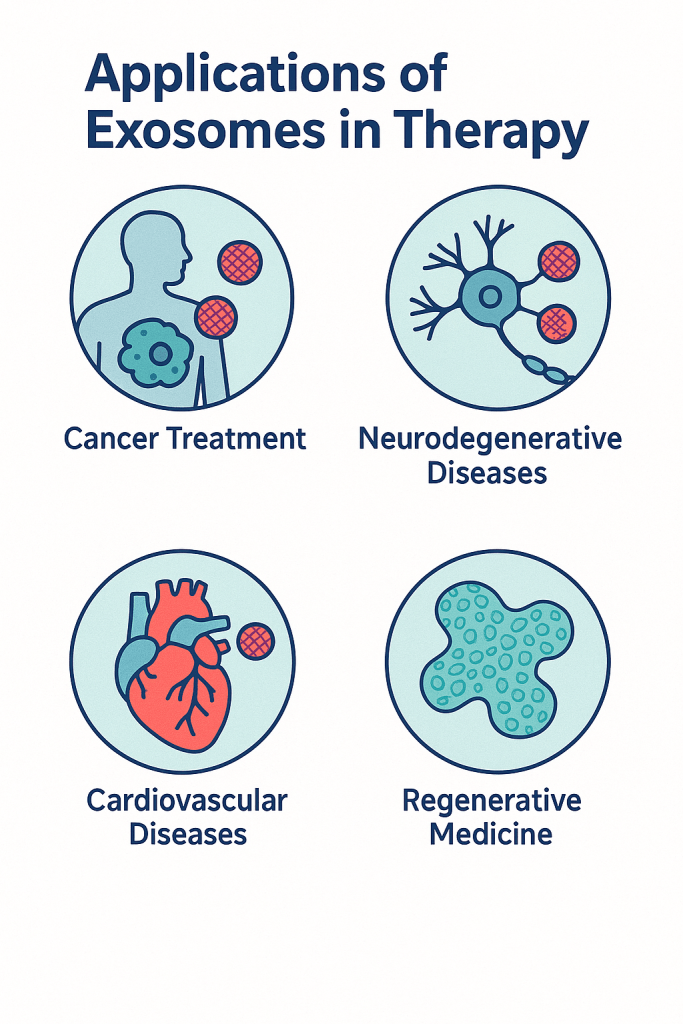
Exosome-based therapies now span a wide range of clinical and translational applications:
- Cancer Treatment: Delivery of chemotherapeutics, immune modulators, or RNA to tumors with reduced systemic toxicity.
- Neurodegenerative Diseases: Ability to cross the blood–brain barrier enables targeted therapy for Alzheimer’s, Parkinson’s, ALS, and brain injury.
- Cardiovascular Disease: Promotion of vascular repair, reduced inflammation, and enhanced recovery after cardiac injury.
- Regenerative Medicine: Improved wound healing, tissue repair, and anti-inflammatory action without full stem-cell transplantation.
These diverse applications highlight exosomes as a universal biological carrier system—capable of transporting complex therapeutic molecules where traditional drugs cannot reach.
Summary: Why Engineered Exosomes Matter
- Programmable biological nanoparticles for targeted therapy
- Able to carry drugs, RNA, proteins, and gene-editing tools
- Surface engineering ensures precise targeting of diseased tissues
- Crosses the blood–brain barrier—ideal for brain therapies
- Lower toxicity and higher precision than conventional delivery systems
- Major potential in cancer, neurology, cardiology, and regeneration
- Positioned to become a cornerstone of future precision medicine